Abstract
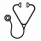
FLT3/ITD mutations occur in approximately 15%-25% of AML in children and young adults and can be amenable to targeting with FLT3 inhibitors. We have demonstrated that those with high ITD allelic ratio (AR) have an adverse prognosis, which can be ameliorated in part by hematopoietic stem cell transplant (HSCT) in CR1. However, even with aggressive therapy only a subset FLT3/ITD (ITD+) patients are cured, suggesting that within this cohort there exists heterogeneity among responses to therapy and overall outcomes. As next generation sequencing has illuminated the distinct and complex genomic profiles of pediatric AML, we hypothesized that presence of cooperating mutations may provide further prognostic information in this group of patients.
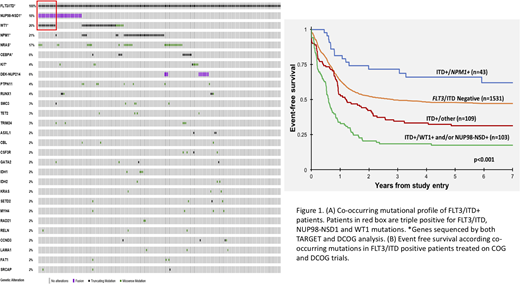
In an international collaborative effort, children and young adults (n=1786; age < 1 month - 29 years, non-APL or Down syndrome) treated on the CCG/COG trials (2961, AAML03P1, AAML0531; n=1472) as well as those treated on the DCOG/BFM/MRC trials (ANLL87, ANLL97, BFM98, BFM04, MRC12, MRC15; n=314) were included in this study. All patients were tested for FLT3/ITD, NPM1, CEBPA, WT1, and NUP98-NSD1 mutations. COG patients underwent either targeted exome or clinical sequencing, while patients in DCOG/BFM/MRC analysis underwent targeted clinical sequencing of hotspot regions in 9 genes. As expected given the heterogeneity of AML, we detected a variety of co-occurring mutations among the ITD+ patients (n=255), ranging from 1-13 (median 2) additional mutations. Overall, we identified 82 different genes with alterations co-occurring with FLT3/ITD mutations. The most prevalent co-occurring mutations were WT1 (26%), NPM1 (21%), NRAS (17%), and CEBPA (6%; Fig 1A). The most common co-occurring fusions were NUP98-NSD1 (18%) and DEK-NUP214 in (6%; Fig 1A). A subset of patients (n=13) harbored FLT3/ITD as well as WT1 and NUP98-NSD1 alternations (ITD+/WT1+/NUP98-NSD1+), referred to as triple positive patients.
We evaluated the prognostic impact of the most common co-occurring mutational profiles as seen in ITD+ children treated on the above cooperative group trials. Clinical outcome of those with FLT3/ITD and favorable (NPM1) or adverse (WT1 or NUP98/NSD1) prognostic factors was contrasted to patients without those risk defining lesions. These presence of the 3 co-occurring mutations added significant prognostic impact when comparing to patients without the 3 mutations (ITD+/other; p<0.001; Fig 1B). Patients with ITD+/other had an event free survival (EFS) of 31% compared to that of 48% in FLT3/ITD negative patients (Fig 1B). Patients with dual ITD/NPM1 mutations (ITD+/NPM1+) had the most favorable outcome with an EFS of 62%, while ITD+ patients harboring either a NUP98-NSD1 and/or WT1 mutation had the most inferior outcome with an EFS of 17%. We sought to further interrogate the effect of the co-occurring mutations in a more homogenously treated cohort of patients treated on COG AAML0531. The ITD+/NPM1+ patients experienced an EFS of 54% which was similar to ITD negative patients. In contrast, ITD+ patients harboring NUP98-NSD1 and/or WT1 experienced an EFS of 24%. Again, the addition of the 3 aberrations to FLT3/ITD demonstrated significant prognostic impact (p<0.001). The triple positive patients experienced an overall survival of <10%. Given the demonstrated significance of AR in risk determination in FLT3/ITD patients, we evaluated the significance of WT1 and NUP98/NSD1 variants in ITD+ patients with a low AR (AR<0.4; n=35). Patients with low AR and WT1 or NUP98/NSD1 variants had a similar dismal outcome as those with higher AR.
Using comprehensive genomic sequencing and outcome evaluation in a large international cooperative study, we found that FLT3/ITD mutations co-occur with a number of other mutations. Importantly, we found that the 3 variants NPM1, WT1, and NUP98-NSD1 can further risk stratify ITD+ patients, regardless of AR. Patients with ITD+/NPM1+ disease experience more favorable outcomes, while ITD+/WT1+ or NUP98-NSD1+ mutant patients do poorly, and triple positive patients have very poor outcomes. This suggests that ITD mutations do not exclusively serve as driving events, rather that co-occurring mutations may impact leukemogenesis and responses to therapy, thus identifying a cohort of patients requiring novel therapeutic approaches to meaningfully improve outcomes.
Gibson:Galen: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract